SterliTECH Tip: Preconditioning of Solvent Resistant Membranes for Organic Solvent Nanofiltration
Solvent recovery is an effective way to help recycle and reuse solvents to reduce fresh solvent consumption. Solvent purification can be achieved efficiently using membrane technology, where a semi-permeable solvent resistant membrane allows the passage of one compound (mostly the solvent), while it retains others (mostly the solute).
Organic Solvent Resistant (OSR) Membranes can be used in organic solvent nanofiltration (OSN), organic solvent reverse osmosis (OSRO), organic solvent forward osmosis (OSFO), organic solvent ultrafiltration (OSU) and pervaporation (PV) for solvent recovery, solute separation and purification in food industry, chemical separation, pharmaceuticals and other fields. The most common applications of solvent resistant membranes in the petrochemical industry include dewaxing of lube oil and methanol recovery in the production of biodiesel. In pharmaceutical production, the technology is mainly used for product concentration, purification and solvent recycling during the synthesis of active pharmaceutical ingredients and high value natural compounds. In the food industry it is particularly used during natural oil processing, for degumming, solvent (hexane) recovery, and deacidification.
Chemically or solvent-resistant membranes are specialized filtration materials designed to withstand exposure to a wide range of chemicals or organic solvents without deteriorating or losing their structural integrity. These membranes are used in industrial applications, including filtration processes where conventional membranes might be compromised by aggressive chemicals or solvents. Their resistance to chemical degradation makes them valuable for separating and purifying substances in challenging environments, for example, strong acid and alkali, as well as different organic solvents ( Alkanes, Aromatics, Alcohols, Ethers, Ketones and Esters).
Preconditioning new flat and spiral wound membranes is discussed in this blog post here, but is preconditioning chemically or solvent-resistant membranes for organic solvent nanofiltration any different?
Preconditioning is important for successful membrane performance which can be enhanced by exposing the membrane to an organic solvent for a certain time before using it for filtration.
Preconditioning can influence the flux through the membrane through the “pore wetting” mechanism. This was seen when a hydrophobic nanofiltration (NF) membrane was preconditioned with hydrophilic solvents (acetone, ethanol and methanol). The membrane was hydrophilized by the treatment and showed high fluxes of ethanol and water. After drying of the acetone preconditioned membrane, the water flux dropped dramatically, indicating restoration of the membrane hydrophobicity so the water molecules could poorly penetrate the membrane pores and wet the pore walls.
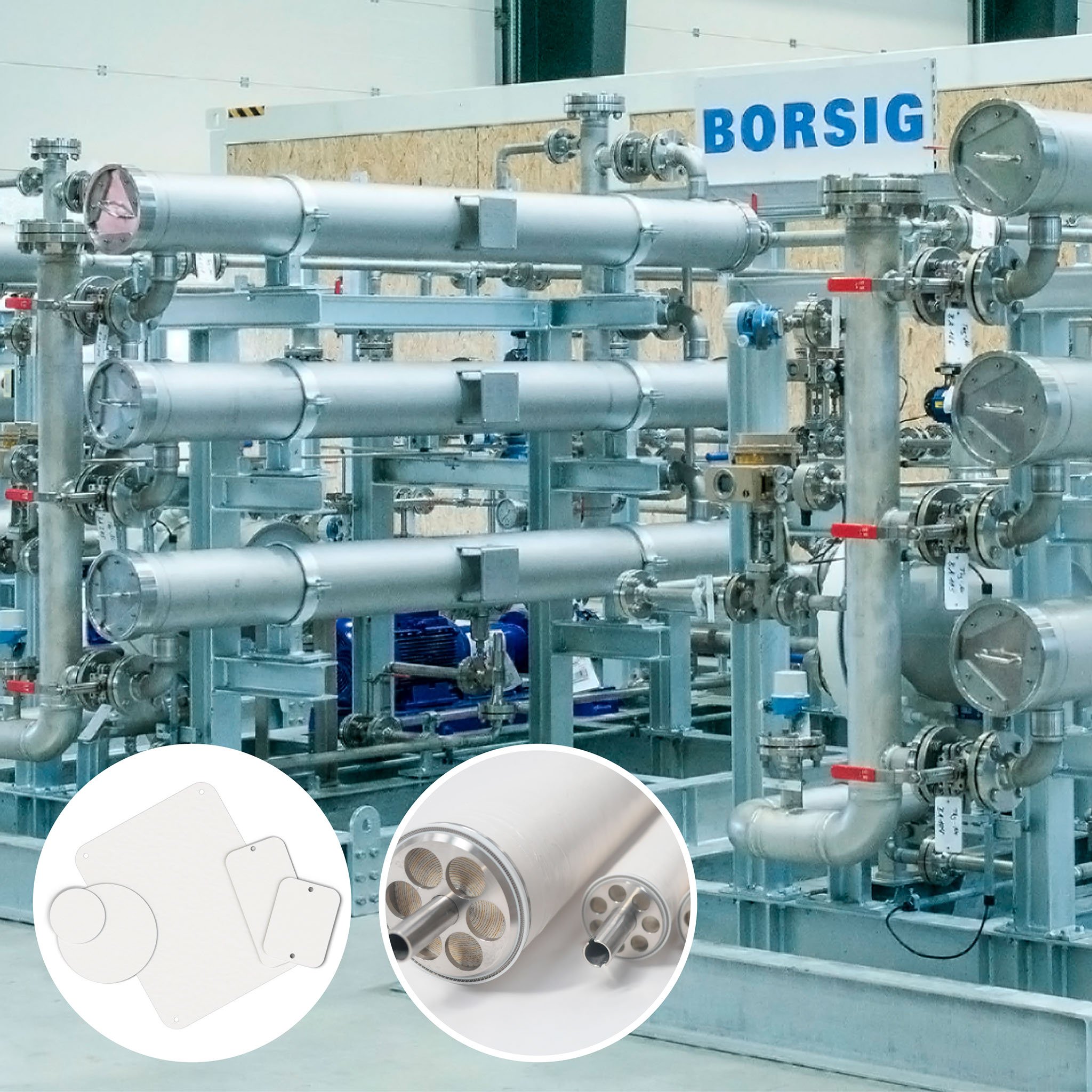
Chemically resistant membranes BORSIG (oNF - organophilic nanofiltration membrane) and Evonik (PuraMem®) require preconditioning before use for the treatment of liquid organic mixtures.
The membrane sample shall be immersed in the pure solvent containing less than 1% w/w (weight in weight) water for at least 2 hours before testing. The conditioning solvent shall be disposed of afterward.
Remember, once wet, it's imperative to prevent any polymeric membrane from drying out. This crucial step ensures that these resilient membranes continue to excel in challenging environments, delivering the highest level of filtration performance.
Preconditioning of solvent resistant membrane is a process that can affect the performance of the membrane in terms of flux and retention. Depending on the type of solvent and the membrane material, preconditioning can have different effects on the membrane structure and properties.
- Most Viewed Blog Articles (5)
- Company News (284)
- Emerging Technologies (64)
- Microbiology and Life Science News (93)
- Water and Fluid Separation News (97)
- Filtration Resources (93)
- Product News (19)

![Join Sterlitech at BIO 2024 [Booth #5558]: Exploring the Future of Biotechnology](https://www.sterlitech.com/media/blog/cache/300x200/magefan_blog/b4.jpeg)




