Waste not, Want not: The NASA Water Filtration Challenge

Water is an essential component for life on Earth, constituting 71% of the planet and about 60% of the human body (1). It’s therefore imperative for terrestrial plants, animals, and humans to have a steady supply of fresh, clean water through either natural or artificial processes. The water cycle is Earth’s natural water recycling system in which water travels through all three phases of matter (solid, liquid, gas), getting desalinated, filtered, and purified in the process. Natural filtration sources function in many different, surprising ways including:
- trapping particulates by hard porous materials like gravel and sand
- filter-feeding shellfish consuming organic and metal contaminants from water
- aquatic plants soaking up excess chemical nutrients and replenishing oxygen (2)
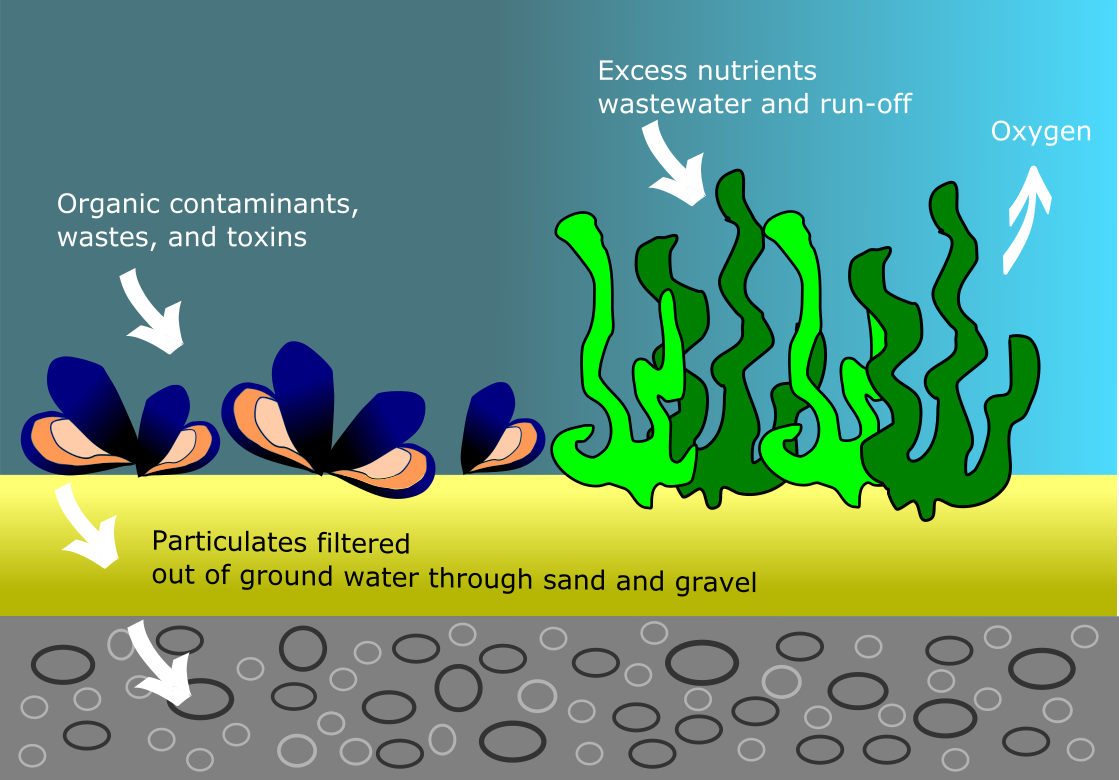
Complex artificial filters used in water treatment plants today are designed to mimic a number of these natural processes, but on a larger and faster scale.
But what about humans living in space? How is urine, sweat, humidity, and wastewater from NASA astronauts living aboarding the International Space Station (ISS) recycled? The water recovery system launched in 2008 resolved this issue, and NASA offers a similar challenge to its youngest scientists.
About The Challenge
The Water Recovery System (WRS), aboard the (ISS) handles all water filtration for astronauts living on the space station, reclaiming up to 90% of the water that astronauts use on a daily basis (3). All the collected moisture feeds into a closed loop system which is passed through a number of physical and chemical filtration processes and then tested by electrical conductivity sensors before being approved for reuse.
The NASA Water Challenge is an inquiry based activity for students in grades 5-12 to design and produce a water filtration device using household materials by following the same process that NASA engineers used to create the ISS WRS (4). The challenge and accompanying lesson plans encourage students to think about the complexities of water filtration by applying critical problem solving skills. During the activity students will propose original designs, collaborate, test their designs, make observations, and collect data.
Access full instructions and detailed lesson plans here.
Materials:
- Balance or scales for weighing
- 250 mL graduated cylinders
- Two empty 0.5 L water bottles
- Scissors
- Paper towels
- 10 cm x 10 cm cheesecloth
- Rubber bands
- Filter media (cotton balls, coffee filters, gravel, sand, uncooked pasta, activated carbon, zeolite)
- Scoop
- Simulated wastewater (1 gal = 2 cups distilled vinegar + yellow food coloring + dust from the floor + ½ cup top soil or sand + handful of hair + water)
- Conductivity testers (optional)
- pH test strips (1-12 range)
Outcomes: The expected outcome of this challenge is to effectively filter the simulated wastewater, removing particulates and color from the dye, and restoring the pH to a neutral range. Students will learn that different combinations of filter materials can have different effects on the final quality of the recycled water. More broadly, the aim is for students to enjoy the scientific process and think creatively to design, execute, and analyze their ideas.
Reference
- The Water in You: Water and the Human Body. Retrieved from https://www.usgs.gov/special-topic/water-science-school/science/water-you-water-and-human-body?qt-science_center_objects=0#qt-science_center_objects
- Parker KT. Natural Materials Used for Water Filtration. 2017 April 24. Retrieved from https://sciencing.com/natural-materials-used-water-filtration-5371122.html
- Water on the Space Station. Retrieved from https://science.nasa.gov/science-news/science-at-nasa/2000/ast02nov_1
- Water Filtration Challenge. Retrieved from https://www.jpl.nasa.gov/edu/teach/activity/water-filtration-challenge
- Most Viewed Blog Articles (5)
- Company News (285)
- Emerging Technologies (64)
- Microbiology and Life Science News (93)
- Water and Fluid Separation News (97)
- Filtration Resources (93)
- Product News (19)

![Join Sterlitech at BIO 2024 [Booth #5558]: Exploring the Future of Biotechnology](https://www.sterlitech.com/media/blog/cache/300x200/magefan_blog/b4.jpeg)



