Using the Sterlitech Schistosome Test Kit
With around 220 million infected in 2017, schistosomiasis (“snail fever”) remains a major public health concern, especially in the endemic regions of Africa which account for about 90% of cases worldwide [1]. Schistosomiasis is caused by tiny, parasitic flatworms carried and released by certain species of freshwater snails. Infection occurs when individuals come into contact with contaminated water. The disease burdens low-income communities in developing regions with poor access to safe sources of water and sanitary facilities [2].
The chronic effects of schistosomiasis can have long-term deleterious impacts on a person’s social and economic well-being [3]. As such, it is vital that the infection be diagnosed as early as possible to avoid the disease progressing to chronic and advanced stages [4].
The World Health Organization (WHO) recommends two methods for the diagnosis of schistosomiasis with accordance to the route of infection: Kato-Katz technique for intestinal infection and Syringe Filtration method for urogenital infection [5]. These two methods are the commonly used in the field to detect and diagnose schistosome infection. The schistosome egg count is the basis for quantifying the magnitude of infection.
Sterlitech’s Role?
Materials and Reagents Required
The materials needed for this procedure are as follows:
- Personal Protective Equipment
- Microscope
- Microscope Slides
- Lugol’s Iodine
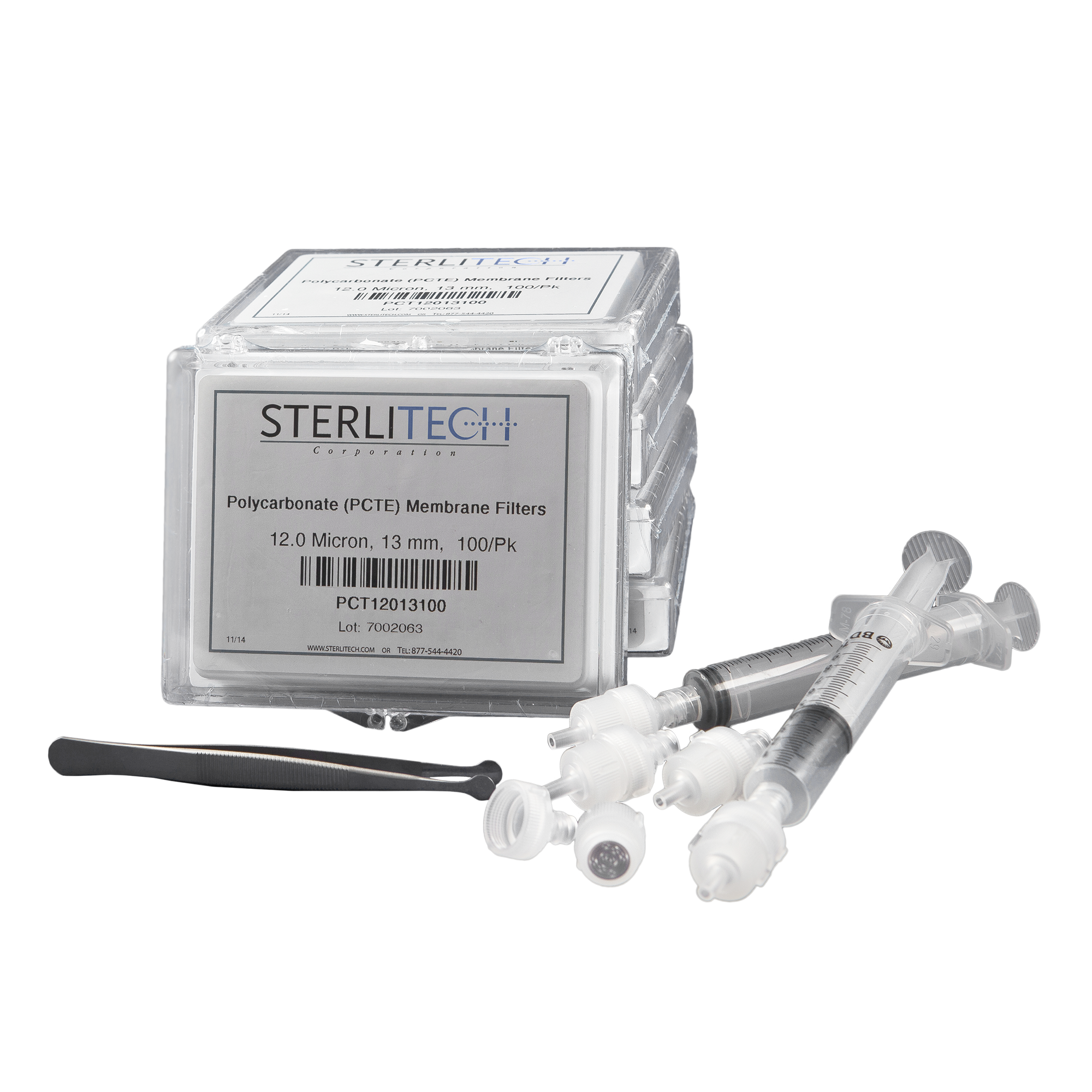
- Schistosome Test Kit, includes:
- 500 polycarbonate (PCTE) disc filters, 13 mm
- 5 filter holders, 13 mm
- 2 syringes, 10 mL capacity
- Membrane Forceps
Procedure:
- Don safety glasses and examination gloves.
- Open the filter holder. Using the forceps, carefully retrieve a new disc filter from its storage box and install in the holder; then securely close the holder. The PCTE membrane filter is thin, translucent and colorless; do not confuse it with the blue separator paper (which is discarded).
- Agitate the urine sample by gently shaking, or by filling and emptying the syringe into the urine sample bottle at least twice.
- Draw 10 milliliters (mL) of the urine sample into the syringe. If 10 mL is not available, draw as much as possible and note the volume.
- Attach the filter holder, as prepared in Step 1, to the outlet of the filled syringe.
- While carefully holding the syringe and attached filter holder vertically over a waste receptacle, gently expel all the urine sample from the syringe through the filter holder. To ensure that the entire urine sample is forced through the filter and that any eggs are well attached to the filter, detach the holder from the empty syringe, draw in a few mL of air, reattach the holder to the syringe, and gently force the air through the holder.
- Detach the filter holder from the syringe and carefully open the holder. Using the forceps, carefully remove the filter from the holder and, while maintaining the same orientation, place it on a clean microscope slide.
- Add one drop of Lugol’s iodine on top of the membrane filter. Wait 15 seconds for the stain to penetrate the eggs. This will impart the eggs with an orange tint that improves visibility under microscopic examination.
- Immediately examine the entire membrane filter using a microscope at low power (typically 10x). Count the total number of eggs on the filter. The infection load is expressed as the number of eggs per 10 mL of urine sample. If the urine sample is less than 10 mL, correct the infection load using the formula below:
| Corrected Infection Load = | No. of Eggs Counted | x 10 |
| Volume (mL) of Urine Sample |
Watch a demo of the Syringe Filtration method here[6]:
Reuse of Schistosome Kit Components
Only the syringes and filter holders can be reused, while the PCTE membranes cannot be reused. Please ensure that no schistosome eggs cling to these devices. To do this, thoroughly rinse the disassembled syringes and filter holders with clean water, preferably purified water, after each use. If desired, detergent and bleach (1% hypochlorite) solutions can be used for washing.
While the membrane filter can be potentially cleaned, it is not practical or cost effective to do so. The time, effort, and materials needed to ensure that the filter is egg-free results in greater cost than simply using a new membrane filter. Also, using a new membrane filter for each test ensures accurate results.
The Fight Against Schistosomiasis
Early diagnosis of schistosome infection is part of a larger strategic drive by WHO which consists of the following: administration of preventive chemotherapy; early diagnosis, treatment, and disease management; control of schistosome vector organisms; and provisions for improved sanitary and hygienic conditions to ensure safe sources of water [7]. In conjunction with aligned efforts from local governments, non-governmental organizations, and pharmaceutical companies, WHO envisions a world free of schistosomiasis by 2025 [7]. To learn more, download our white paper.
References
- World Health Organization. (2018). Weekly epidemiological record (WHO Publication No.50, Vol. 93). Retrieved from https://www.who.int/wer/en/
- Hotez, P.J. (2013). NTDs V.2.0: “Blue Marble Health”—Neglected Tropical Disease Control and Elimination in a Shifting Health Policy Landscape. PLoS Neglected Tropical Diseases 7(11), e2570. doi:10.1371/journal.pntd.0002570
- Bruun, B., & Aagaard-Hansen, J. (2008). The social context of schistosomiasis and its control: an introduction and annotated bibliography. Geneva: World Health Organization in behalf of the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). Retrieved from https://www.who.int/tdr/publications/documents/social-context-schistosomiasis.pdf
- Weerakoon, K.G.A.D., Gobert, G.N., Cai, P., & McManus, D.P. (2015). Advances in the Diagnosis of Human Schistosomiasis. Clinical Microbiology Reviews, 28(4), 939-967. doi:10.1128/CMR.00137-14
- World Health Organization. (1991). Basic laboratory methods in medical parasitology. Geneva: World Health Organization.
- World Health Organization. (n.d.). Basic laboratory methods in human parasitology: examination of faecal and urine specimens; Part 07 – Urine tests for schistosomiasis [Video file]. Retrieved from https://www.who.int/neglected_diseases/preventive_chemotherapy/Basic_Lab_methods_in_human_parasitology/en/index8.html
- Crompton, D.W.T. (Ed.). (2012). Accelerating work to overcome the global impact of neglected tropical diseases – A roadmap for implementation. Geneva: WHO Press.

![Join Sterlitech at BIO 2024 [Booth #5558]: Exploring the Future of Biotechnology](https://www.sterlitech.com/media/blog/cache/300x200/magefan_blog/b4.jpeg)



