Summary
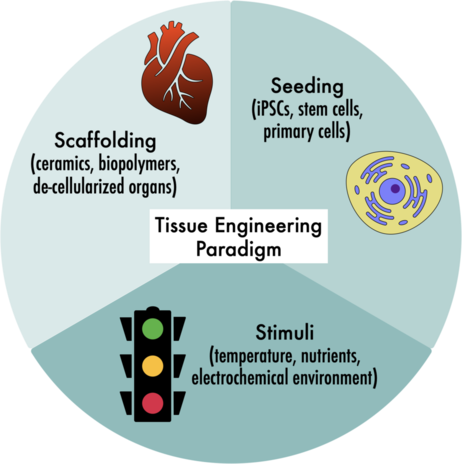
Tissue engineering is a broad, interdisciplinary field that has contributed significant progress to regenerative medicine, drug discovery, and disease pathology. While tissue engineering (TE) is complex and highly variable in practice, the TE paradigm relies on three essential steps, namely scaffolding, seeding, and stimuli.
The Tissue Engineering Paradigm
A paradigm is a model that draws from a specific set of theories, methods, and standards. The Tissue Engineering Paradigm encompasses historical and future efforts to cultivate living tissue outside the body--from scratch. For example, developing a functional organ, such as a heart, requires the interaction of multiple cell types with specifically timed chemical and physical signals all within a complex and delicate 3D framework (1).
Step 1: Scaffolding the new tissue shape and structure
Scaffolding is a technologically complex component of the TE paradigm. The scaffold should closely replicate fine structural features in the native tissue. It also has to promote cells to adhere, spread, and mature without causing adverse reactions (2). Ceramics are a common choice for scaffolding hard tissues, while biocompatible polymers or gels are used for soft tissues, and new scaffolding materials are still being developed and discovered (2). In fact, a research group from the University of Ottawa has even proposed using soda bread as a cost effective and easy to produce tissue scaffold (3)
Step 2: Seeding the scaffold with living cells
For regenerative medicine, cells should be isolated from a human patient and not an animal model (1). The cell source can be adult primary cells from the same tissue, for example skin cells from a biopsy could be used to seed a TE skin graft (2). However, adult primary cells are slow growing, difficult to isolate, and intensely specific to their function (1). Stem cells are more versatile because they are self-renewing and they can differentiate into multiple cell types (2). For a long time, embryonic stem cells were the only source of undifferentiated cells, but recent discoveries proved that some adult cell types can also be reverted back to stem cells (1, 4). These induced pluripotent stem cells (iPSCs) are currently being used for tissue engineering, drug discovery, and disease studies in many tissue types (4).
Step 3: Sending the right signals with physiochemical stimuli
Cells inside the body are in constant contact with each other and their outside environment. Chemical signals like an influx of Ca2+ to initiate muscle contraction and physical signals like temperature activated immune cells (5) have strong inducible effects that help our tissues to function. Other processes controlled by known and unknown biochemical stimuli include import/export across membranes and cell shape, size, growth, and death (2). Incubating TE constructs in bioreactors that modulate conditions to match the dynamic environment inside a body can give engineers fine-tuned control over tissue development (2).
Putting it all together
Significant advances in the TE paradigm include the development of novel scaffolding materials, the emergence of induced pluripotent stem cells, and bioreactor technology that gives greater control over cell culture conditions (2, 3). However, challenges remain in all three steps of the TE paradigm, largely stemming from the sheer complexity of mimicking a biological system. Tissue engineering is a rapidly evolving field, and the tissue engineering paradigm is likely to be at the center of a new generation of bioartificial science for years to come.
References
- Benam, K.G., Dauth, S., Hassell, B. et al. Engineered In Vitro Disease Models. Annu. Rev. Pathol. Mech. Dis. 10 (2015), 195-262. https://doi.org/10.1146/annurev-pathol-012414-040418
- Caddeo, S., Boffito, M., Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 5:40 (2017), 1-40. https://doi.org/10.3389/fbioe.2017.00040
- Holmes, JT., Jaberansari, Z., Collins, W., et al. Homemade Bread: Repurposing an Ancient Technology for Low Cost in vitro Tissue Engineering. bioRxiv (2020). https://doi.org/10.1101/2020.11.13.353698
- Takahashi K., Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:4 (2006) 663-676. https://doi-org.oca.ucsc.edu/10.1016/j.cell.2006.07.024
- Federation of American Societies for Experimental Biology. Elevated body temperature helps certain types of immune cells to work better, evidence suggests. ScienceDaily. (2011). Retrieved from https://www.sciencedaily.com/releases/2011/11/111101130200.htm

![Join Sterlitech at BIO 2024 [Booth #5558]: Exploring the Future of Biotechnology](https://www.sterlitech.com/media/magefan_blog/b4.jpeg)

