Liposome’s Structure and Extrusion: Introduction, Method, and Application
Liposomes(1) are defined as closed, spherical lipid bilayer vesicles(2) that form an internal cavity and serve as nanocarriers(2) of aqueous solutions and a wide range of bioactive molecules. It forms when tissue is damaged resulting in exposure of the lipid bilayer that folds back on itself, encapsulating(2) a small packet of whatever solution it forms in it. These are then used in studying cell membranes and organelles by embedding different proteins and solutions into the lipid bilayer and mostly specific to drug delivery(3), gene research and cosmetics(1)
Liposome extrusion(1) is crucial in producing unilamellar vesicles with a homogeneous size distribution and the most efficient method of nanosizing liposomes. The homogeneity of the liposome sizes is crucial to prolong the liposome circulation time(2) and maintain its strength during storage. Moreover, liposomes with consistent size characteristics can exhibit better control over drug release rates(4) and increased cellular uptake.
Method and Application
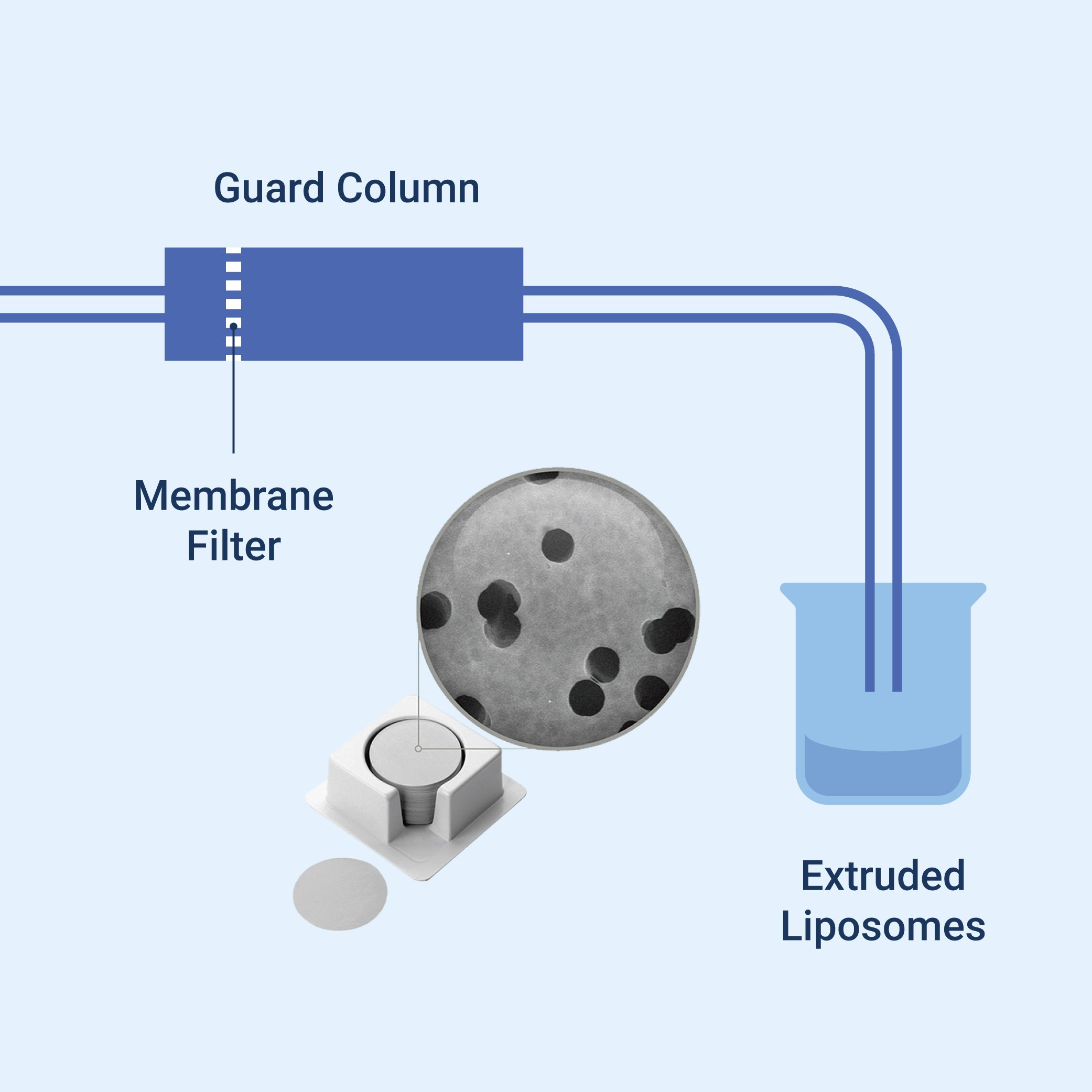
Liposome extrusion(1) can be done in two primary methods: Manual and Automated. Both of which are a process wherein the liposome suspension is passed through a membrane filter of defined pore size. Prior to extrusion through the final pore size, the suspensions are disrupted either by several freeze-thaw cycles(1) or by prefiltering the suspension through a larger pore size which helps to prevent the membrane to accumulate other unwanted material and improves the uniformity of the size distribution after the extrusion procedure.
During manual extrusion(2), the liposome suspension passed through the polycarbonate membrane by using a syringe or an extruder device. The pore size of the utilized polycarbonate membrane(5) filter will determine the resulting liposome size. This manual procedure is suitable for small scale research or production since it was more economical and very straightforward.
However, if automated, there is an extruder, a machine equipped with a pump which pushes fluids through the membranes. This machine Is computer-controlled that will check and regulate the pressure and temperature during the process. This automated procedure(6) will provide greater reproducibility and is ideal for large-scale production and more complex formulation.
There are few factors(1) that needs to be considered for a successful extrusion such as the following:
- Membrane selection(1) (material and pore size) - Commonly used membranes include polycarbonate(7), track-etched, and anodized aluminum oxide(5). While the pore size affects the polydispersity index, which is a measure of the liposomes' size distribution.
- Temperature(1) – liposome extrusion that was performed on higher temperature above the lipid transition temperature are steadier and prevents lipid coagulation/accumulation.
- Pressure(1) – controlling pressure aids on achieving homogeneity of the desired liposome size and minimizing membrane damage
- Extrusion cycle / flow rate(1)– repeating extrusion cycle might improve the homogeneity of liposome size, however, may result in destabilization and membrane rupture if done excessively. Increase in the flow rate of the extrusion process decreased the size of extruded liposomes, but the size homogeneity was negatively impacted.
Sterlitech product recommendations
PCTE (Polycarbonate) Membranes - Membrane Disc Filters | Sterlite (sterlitech.com)
References:
[1] Ong, S.G. et al. (no date) Evaluation of extrusion technique for nanosizing liposomes. Available at: https://pdfs.semanticscholar.org/c185/35e3697aee0aae3f0f46fd5946f8e4717362.pdf.
[2] Guo, P., Huang, J. and Zhao, Y. (no date) Nanomaterial preparation by extrusion through nanoporous membranes. Available at: https://www-leland.stanford.edu/group/Zarelab/publinks/985.pdf
[3] Allen, T.M. et al. (2012) Liposomal drug delivery systems: From concept to clinical applications, Advanced Drug Delivery Reviews. Elsevier. Available at: https://www.sciencedirect.com/science/article/pii/S0169409X12002980.
[4] Andra, V.V.S.N.L. et al. (2022) A comprehensive review on novel liposomal methodologies, commercial formulations, clinical trials and patents, BioNanoScience. U.S. National Library of Medicine. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8790012/.
[5] Olson, F. et al. (2001) Filter extrusion of liposomes using different devices: Comparison of liposome size, encapsulation efficiency, and process characteristics, International Journal of Pharmaceutics. Elsevier. Available at: https://www.sciencedirect.com/science/article/pii/S0378517301007219 .
[6] Shah, V. et al. (2019) Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes, Nanomedicine: Nanotechnology, Biology and Medicine. Elsevier. Available at: https://www.sciencedirect.com/science/article/pii/S1549963419300504
[7] Choudhury, A. et al. (1970) Liposome: A carrier for effective drug delivery, Journal of Applied Pharmaceutical Research. Creative Pharma Assent. Available at: https://www.neliti.com/publications/320114/liposome-a-carrier-for-effective-drug-delivery.
Additional references:
Arpita, S., Kumar, S.V. and Amresh, G. (no date) (PDF) liposomes – a review - researchgate. Available at: https://www.researchgate.net/publication/347685063_LIPOSOMES_-_A_REVIEW
Berger, N. et al. (2001) Filter extrusion of liposomes using different devices: Comparison of liposome size, encapsulation efficiency, and process characteristics, International Journal of Pharmaceutics. Elsevier. Available at: https://www.sciencedirect.com/science/article/pii/S0378517301007219.
Dhawan, V., Holm, R. and Nagarsenker, M. (no date) Liposomes: Advancements and innovation in the manufacturing process. Available at: https://strathprints.strath.ac.uk/75205/1/Shah_etal_ADDR_2020_Liposomes_advancements_and_innovation_manufacturing_process.pdf
Hope, M.J. et al. (no date) Production of large unilamellar vesicles by a rapid extrusion procedure: Characterization of size distribution, trapped volume and ability to maintain a membrane potential, Biochimica et biophysica acta. U.S. National Library of Medicine. Available at: https://pubmed.ncbi.nlm.nih.gov/23008845/
Nsairat , H.A., Khater, D.B. and Sayed, U. (no date) Liposomes: Structure, composition, types, and clinical applications, Heliyon. U.S. National Library of Medicine. Available at: https://pubmed.ncbi.nlm.nih.gov/35600452/
Shah, S. et al. (2020) Liposomes: Advancements and innovation in the manufacturing process, Advanced Drug Delivery Reviews. Elsevier. Available at: https://www.sciencedirect.com/science/article/pii/S0169409X20300788

![Join Sterlitech at BIO 2024 [Booth #5558]: Exploring the Future of Biotechnology](https://www.sterlitech.com/media/blog/cache/300x200/magefan_blog/b4.jpeg)



