A Sustainable Future Through Batteries
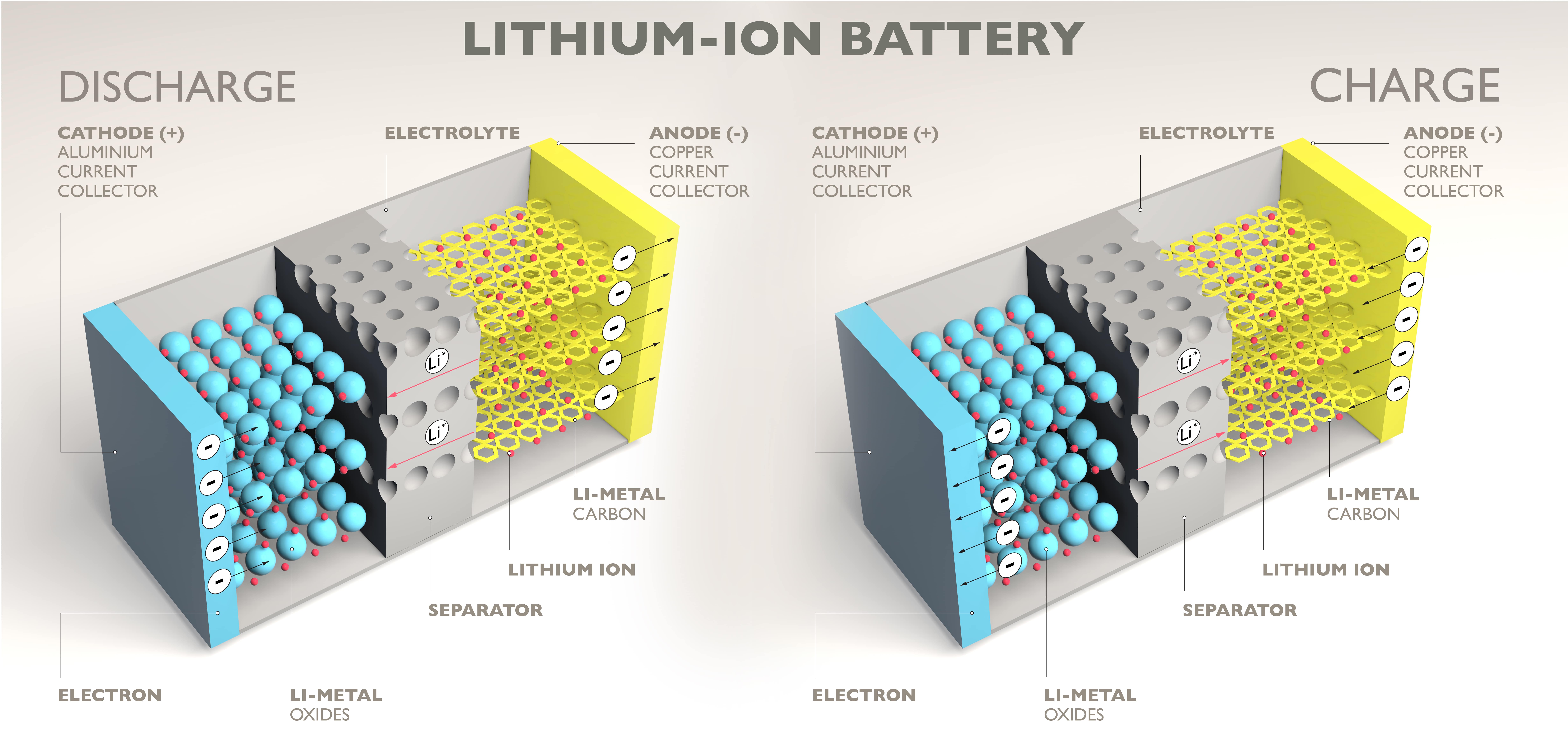
From their use in portable electronics and medical devices to electric vehicles, lithium (Li)-ion batteries have revolutionized our daily lives. Appropriately, the 2019 Nobel Prize for Chemistry has been awarded to M. Stanley Whittingham, John Goodenough, and Akira Yoshino for their major contributions in the development of the Li-ion battery.[1]
So, what’s special about lithium? Lithium is the lightest metal in the periodic table and has a very low standard reduction potential, making it a useful candidate for small and lightweight high-capacity batteries.[2][3] However, its high reactivity with water and air limits its commercial application. The Nobel Laureates spent nearly a decade of compounded work to overcome this limitation.
In 1976, M. Stanley Whittingham proposed and created the first lithium battery. His design consisted of a titanium disulfide cathode where lithium ions could enter and exit through a process called intercalation.[4] Intercalation enhanced the cycle of discharging and charging and would become the basis for later designs.[2] However, his use of lithium metal as the anode proved to be a safety hazard, so the intercalation of lithium ions in graphite anode was studied to replace lithium metal anode.[5] The concept was not so simple, though, and the replacement at first resulted in lower cell potential.[2] Meanwhile, John Goodenough focused on improving the cathode design and in 1979, he made a cell using an intercalated cobalt oxide cathode. His design showed a higher cell potential of 4-5 volts (V) compared to the 2.5 V potential from Whittingham’s design.[6] Once a higher cell potential could be achieved, the work could then focus on making graphite intercalation possible. In 1985, a group led by Akira Yoshino achieved this by using petroleum coke, enabling a lithium battery design that won’t cause fire or explosion even when damaged.[7] These compounded discoveries eventually led to the commercialization of the Li-ion battery in 1991.[2] Since then, the Li-ion battery has been continually improved and become a ubiquitous component in modern electronic devices.
To function, a battery needs a cathode and an anode (collectively called electrodes), a separator, and an electrolyte. Continuous development of safer and higher energy capacity designs revolves around these components.[8] Sterlitech Membrane Filters have been used in this endeavor as substrates for electrodes, material for separators, and purification in electrode fabrication.[9][10][11][12][13][14] In one recent example, a silver membrane cathode reinforced with samarium-doped ceria showed a promising application in solid oxide fuel cells (SOFC).[15]
The continuous improvement and use of batteries is essential to energy sustainability. While we have the current technologies to collect solar and wind power, they are rather intermittent and cannot replace fossil fuel in the grid level of power distribution—yet.[16] To integrate renewable sources into the grid, an effective energy storage system is needed.[17][18] The use of Li-ion batteries in electric vehicles and their capability of storing renewable energy shows promise. With further advancement to reduce cost and enhance battery efficiency at megawatt capacities, we can greatly increase renewable energy infrastructure and move closer to carbon neutrality.
References
[1] The Royal Swedish Academy of Sciences. (2019, October 09). The Nobel Prize in Chemistry 2019 [Press Release]. Retrieved from
[2] Ramström, O. (2019, October 09). Scientific Background on the Nobel Prize in Chemistry 2019. Stockholm: The Royal Swedish Academy of Sciences. Retrieved from https://www.nobelprize.org/
[3] Liu, B., Zhang, J.G., & Xu, W. (2018). Advancing Lithium Metal Batteries. Joule, 2(5), 833-845. Cambridge: Cell Press. doi: 10.1016/j.joule.2018.03.008
[4] Whittingham, M. (1976). Electrical Energy Storage and Intercalation Chemistry. Science, 192, 1126-1127.
[5] Besenhard, J.O., & Eichinger, G. (1976). High energy density lithium cells: Part I. Electrolytes and Anodes. J. Electroanal. Chem. Interfacial Electrochem., 68, 1-18.
[6] Mishuzima, K., Jones, P.C., Wiseman, P.J., & Goodenough, J.B. (1980). LixCoO2 (0<x<-1): A new cathode material for batteries of high energy density. Materials Research Bulletin, 15(6), 783-789. Amsterdam: Elsevier. doi: 10.1016/0025-5408(80)90012-4
[7] Yoshino, A., Sanechika, K., & Nakajima, T. (1987). US Patent No. 4,668,595. Washington, DC: U.S. Patent and Trademark Office.
[8] Deng, D. (2015). Li-ion batteries: basics, progress, and challenges. Energy Science & Engineering, 3(5), 385-418. New Jersey: John Wiley & Sons. doi: 10.1002/ese3.95
[9] Bidaut, F. & Kucernak, A. (2011). Cathode development for alkaline fuel cells based on a porous silver membrane. Journal of Power Sources, 196(11), 4950-4956. doi: 10.1016/j.jpowsour.2011.02.008
[10] Chaduang, S., Lao-atiman, W., & Kheawhom, S. Improving Performance and Cyclability of Printed Flexible Zinc-Air Batteries Using Carbopol Gel Electrolyte. Journal of The Electrochemical Society, 77(1), 55-62. New Jersey: ECS. doi: 10.1149/07701.0055ecst
[11] Kim, T.Y., Jung, G., Yoo, S., Suh, K.W., & Ruoff, R.S. (2013). Activated Graphene-Based Carbons as Supercapacitor Electrodes with Macro- and Mesopores. ACS Nano, 7(8), 6899-6905. doi: 10.1021/nn402077v
[12] Chun et al. (2015). Design of aqueous redox-enhanced electrochemical capacitors with high specific energies and slow self-discharge. Nature Communications, 6, 7818. doi: 10.1038/ncomms8818
[13] Dyatkin, B., Presser, V., Heon, M., Likatskaya, M.R., Beidaghi, M., & Gogotsi, Y. (2013). Development of a Green Supercapacitor Composed Entirely of Environmentally Friendly Materials. ChemSusChem, 6(12), 2269-2280. https://doi.org/10.1002/cssc.201300852
[14] Klein, M.J., Goossens, K., Bielawski, C.W., & Manthiram, A. (2016). Elucidating the Electrochemical Activity of Electrolyte-Insoluble Polysulfide Species in Lithium-Sulfur Batteries. Journal of the Electrochemical Society, 163(9), A2109-A2116. New Jersey: ECS. doi: 10.1149/2.0051610jes
[15] Lee, T.H., Baek, J.D., Fan, L., Wiria, F.E., Su, P.C., Lee, S.H. (2018). SDC-Infiltrated Microporous Silver Membrane with Superior Resistance to Thermal Agglomeration for Cathode-Supported Solid Oxide Fuel Cells. Energies, 11, 2181. Basel: MDPI. doi:10.3390/en11092181
[16] Asian Development Bank. (2018). Handbook on Battery Energy Storage System. Retrieved from https://www.adb.org/
[17] Hart, D.M., Bonvillian, W.B., & Austin, N. (2018). Energy Storage for the Grid: Policy Options for Sustaining Innovation (MITEI-WP-2018-04). Retrieved from the MIT Energy Initiative website: https://energy.mit.edu/
[18] International Renewable Energy Agency. (2015). Battery Storage for Renewables: Market Status and Technology Outlook. Retrieved from https://www.irena.org/

![Join Sterlitech at BIO 2024 [Booth #5558]: Exploring the Future of Biotechnology](https://www.sterlitech.com/media/blog/cache/300x200/magefan_blog/b4.jpeg)



